Abstract
Background: Ponatinib and blinatumomab are both highly effective in Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). The combination of these two agents may lead to deep and durable responses, thereby reducing the need for both chemotherapy and allogeneic stem cell transplant (ASCT) in first remission.
Methods: In this phase II study, adults with newly diagnosed (ND) Ph+ ALL, relapsed/refractory (R/R) Ph+ ALL, or chronic myeloid leukemia in lymphoid blast phase (CML-LBP) were eligible. Patients (pts) were required to have a performance status of ≤2, total bilirubin ≤2x the upper limit of normal (ULN), and alanine aminotransferase and aspartate aminotransferase ≤3x the ULN. Pts with uncontrolled cardiovascular disease or clinically significant central nervous system (CNS) comorbidities (except for CNS leukemia) were excluded. Pts received up to 5 cycles of blinatumomab as a continuous infusion at standard doses. Ponatinib 30mg daily was given during cycle 1. Ponatinib was decreased to 15mg daily once a complete molecular response (CMR) was achieved. After completion of blinatumomab, ponatinib was continued for at least 5 years in responding pts. Twelve doses of prophylactic intrathecal chemotherapy with alternating cytarabine and methotrexate were administered. For pts with ND Ph+ ALL, the primary endpoint was the CMR rate. For pts with R/R Ph+ ALL, the primary endpoint was the overall response rate (defined as the composite of CR/CRi).
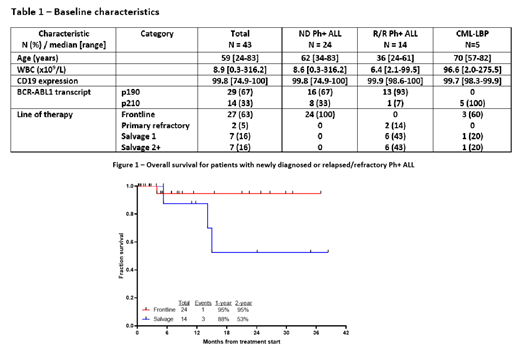
Results: Between February 2018 and July 2021, 43 were treated (24 ND, 14 R/R and 5 CML-LBP). Baseline characteristics are shown in Table 1. The median age of the ND and R/R Ph+ ALL cohorts were 60 years (range, 34-83) and 38 years (range, 24-61), respectively. BCR-ABL1 transcripts were p190 in 67% of pts in the ND cohort and 93% in the R/R cohort. 43% of pts in the R/R Ph+ ALL cohort were in salvage 2 or beyond.
Among 32 pts evaluable for morphologic response, all but 1 pt (97%) responded. Notably, the one non-responding pt had R/R Ph+ ALL and had previously received ponatinib in an earlier salvage regimen. The CR/CRi rate was 100% for ND pts, 91% for R/R pts, and 100% CML-LBP pts. 84% of responding pts achieved CMR (91% in the ND cohort, 91% in the R/R cohort, and 40% in the CML-LBP cohort). The CMR rates after 1 cycle were 64%, 82% and 20% in these 3 groups, respectively. Among 10 pts in the ND cohort who had peripheral blood PCR for BCR-ABL1 evaluated on day 7 of cycle 1, 3 (30%) had already achieved CMR at this early timepoint; 5 out of 13 evaluable patients (38%) had achieved CMR by days 14-21 of cycle 1
The median follow-up is 9 months (range, 1-38+ months). Among the 24 pts in the ND cohort, 1 pt died in CR, and the rest are all in ongoing response without HSCT in first remission, with a median CR duration of 8 months (range, 1-36+ months). The estimated 2-year EFS and OS for the ND cohort is 95% (Figure 1). Among the 14 pts in the R/R cohort, 2 are too early for response evaluation, 1 did not respond, 4 underwent ASCT (3 of whom are still alive without relapse and 1 of whom relapsed post-ASCT and died), 2 did not undergo ASCT and subsequently relapsed, 1 died in CR, and 4 are in ongoing response without ASCT. The estimated 2-year EFS and OS for the R/R cohort are 53% and 39%, respectively (Figure 1). Among the 5 pts in the CML-LBP cohort, 2 relapsed (1 of whom converted to myeloid immunophenotype) and 3 are in ongoing response without ASCT.
The combination treatment was well-tolerated, and most side effects were grade 1-2 and were consistent with the known toxicity profile of the two agents individually. Two pts discontinued ponatinib due to toxicity (1 due to stroke and 1 due to DVT). No pts discontinued blinatumomab due to toxicity. No early deaths were observed.
Conclusion: The chemotherapy-free combination of ponatinib and blinatumomab is a safe and effective regimen in both ND and R/R Ph+ ALL, as well as in CML-LBP. Given the particularly favorable outcomes of pts with ND Ph+ ALL who were not transplanted in first remission, these data suggest that this regimen may serve as an effective transplant-sparing regimen in this population.
Short: Novartis: Honoraria; Jazz Pharmaceuticals: Consultancy; AstraZeneca: Consultancy; NGMBio: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Kantarjian: Ascentage: Research Funding; BMS: Research Funding; AbbVie: Honoraria, Research Funding; Astra Zeneca: Honoraria; Pfizer: Honoraria, Research Funding; Jazz: Research Funding; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; Astellas Health: Honoraria; Amgen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; Aptitude Health: Honoraria; NOVA Research: Honoraria; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Konopleva: Ascentage: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; KisoJi: Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Cellectis: Other: grant support; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding. Jain: Aprea Therapeutics: Research Funding; Precision Biosciences: Honoraria, Research Funding; Beigene: Honoraria; TG Therapeutics: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Pfizer: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Janssen: Honoraria; ADC Therapeutics: Honoraria, Research Funding; Fate Therapeutics: Research Funding; Servier: Honoraria, Research Funding; Incyte: Research Funding; Genentech: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Pharmacyclics: Research Funding; AbbVie: Honoraria, Research Funding. Ravandi: Prelude: Research Funding; Agios: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; AstraZeneca: Honoraria; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria; Xencor: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Borthakur: University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; Ryvu: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; ArgenX: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sasaki: Novartis: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees. Issa: Kura Oncology: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding. Alvarado: Daiichi-Sankyo: Research Funding; Jazz Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; FibroGen: Research Funding; CytomX Therapeutics: Consultancy; BerGenBio: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Consultancy, Research Funding. Pemmaraju: Celgene Corporation: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; Roche Diagnostics: Consultancy; Cellectis S.A. ADR: Other, Research Funding; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; CareDx, Inc.: Consultancy; Sager Strong Foundation: Other; Plexxicon: Other, Research Funding; Incyte: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Springer Science + Business Media: Other; Aptitude Health: Consultancy; Daiichi Sankyo, Inc.: Other, Research Funding; Affymetrix: Consultancy, Research Funding; Samus: Other, Research Funding; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Clearview Healthcare Partners: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; MustangBio: Consultancy, Other; LFB Biotechnologies: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. DiNardo: Takeda: Honoraria; Novartis: Honoraria; ImmuneOnc: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Forma: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Agios/Servier: Consultancy, Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding.
Blinatumomab and ponatinib as frontline therapy for Ph+ ALL
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal